Researchers are moving a step closer to a definite road map for building layer-by-layer (LbL) assembled polyelectrolyte films, thanks to the Liquids Reflectometer at the Spallation Neutron Source. Faster at data collecting than any other neutron instrument at ORNL, the Liquids Reflectometer has successfully taken snapshots, in close to real time, of how researchers can change the multilayered structures for different applications when they modify the structure and function parameters.
“Being able to control these properties, understanding how what you do to the materials affects their properties, is very helpful. This allows you to apply them to situations where interacting with an environment, whether in a biological context or any other kind of water-soluble context,” says John Ankner, lead scientist for the Liquids Reflectometer.
“The result is the forming of surface films, useful for coatings for biomedical implants and devices, for controlling adhesion of biological molecules and for controlled delivery of therapeutic molecules from surfaces,” says Svetlana Sukhishvili, lead chemist on the project.
“Medical doctors often prefer to deliver multiple therapeutic compounds from the coatings, in a time-resolved manner,” Sukhishvili says. “So material scientists need to learn how to build coatings in which polymer layering will not be compromised upon exposure of such films to normal physiological conditions.” “The whole idea of a study in which several parameters are systematically altered is that one can map out the whole range of structures versus such parameters as charge and bulkiness of the group. Then, for a given application, one can say, ‘This work really sets a road map for how to get started with synthesizing polyelectrolyte materials for specific applications,’ ” Ankner says. “Ok, this one, the one that is 30% charged, this methylated material is going to be what we want to use for a particular application.”
In this research, two types of polyelectrolytes of opposite charge are assembled at surfaces in a sequential way, using the LbL assembly technique.
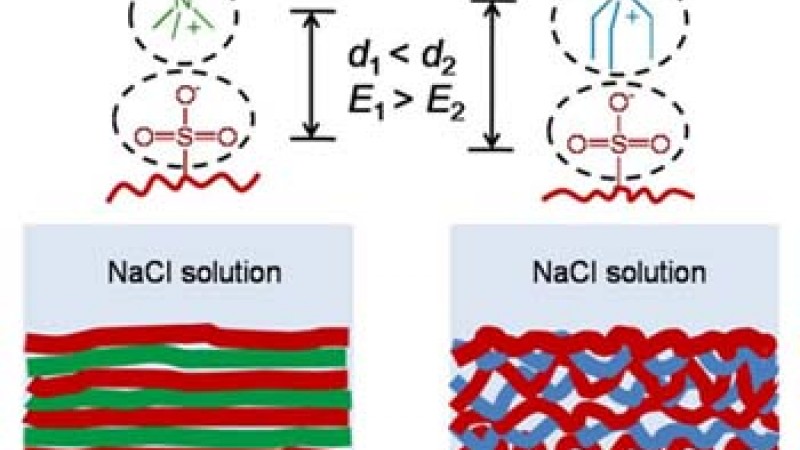
The polyelectrolyte chains in the films are stitched together through what is called ionic pairing and are arranged within fuzzy, ultrathin layers that lie parallel to a solid surface. Film layering can be compromised, however, during exposure of these films to aqueous solutions containing salt ions (i.e., conditions that imitate real life). The salt ions act to weaken the ionic pairing that binds the polyelectrolyte layers together.
A research collaboration of Ankner, Sukhishvili of the Stevens Institute of Technology in New Jersey, and her student Li Xu looked at the effects of changing the charge density and the blocking of access to a reactive site by nearby groups on the layering of two types of LbL films. The films were composed of positively charged variants of PDMA, a methyl polymer and PDEA, an ethyl polymer. The other component of both systems is the ion exchanger polystyrene sulfonate (PSS), which features a fixed negative charge.
To begin, a silicon substrate was dipped into solutions of PDMA and PDEA in dilute sodium chloride for a fixed time. Depending on the deposition time and the concentration of the solution, a nanometer-thick monolayer of the polymer adsorbs to the silicon surface. The film buildup is continued by depositing a layer of PSS, and then the cycle is repeated. The PDMA (methyl)/PSS and PDEA (ethyl)/PSS films were then annealed in varying concentrations of sodium chloride.
The chemists wanted to know if in these multi-layer, cake-like assemblies, the structure can be systematically altered by varying the salt concentration; the time in solution; and ultimately, by varying other environmental parameters such as temperature or pH.
The neutron reflectivity of such layered films will exhibit features indicative of the quality of the layering, in particular the concentration of the layers and how intermixed they are with adjacent layers. In this research, neutron reflectivity data from films built from 10%, 40%, and 100% charged PDMA or PDEA polyelectrolytes and 100% charged PSS were quantitatively compared to different layered arrangements until the models produced reflectivity patterns matching those of the data.
Subsequent annealing in varying sodium chloride solutions over 2, 6, and 12 hour time periods allowed researchers to observe whether the initial well-defined layers intermixed and, if they did, how quickly they intermixed as a result of the enhanced diffusion of polymer chains.
“The instrument is just ideal for parametric studies,” Ankner says. We varied the charge of the monomer units in assembled polymers; we varied the architecture of the polymers, how many carbons, i.e., are the materials bulky or compact; and we were able to vary the salt concentration in the external solution.
“So we actually probed a three-dimensional space of parameters. There are a lot of measurements that went into this, and the most interesting stuff was picked out for presentation in our paper.
“Since this instrument is faster at collecting data than any other, the researchers were able to do this experiment in one visit. We are still digesting the data from when these guys came, more than a year ago, and there is a second paper that is going to come out soon from this same visit.”
“So what we have now is a peanut butter and jelly sandwich. Then we will start putting in raisins and nuts and other things. We are starting to move now from the road map of the structure/function relationship to specific initial applications that would be the first steps towards people actually using these materials for new applications.”
This work was supported by the National Science Foundation under Award DMR-0906474. Research conducted at the Spallation Neutron Source was funded by the U.S. Department of Energy Office of Basic Energy Sciences.
Published Work
L. Xu, J Ankner, and S. Sukhishvili, “Steric Effects in Ionic Pairing and Polyelectrolyte Interdiffusion within Multilayered Films: A Neutron Reflectometry Study,” Macromolecules 44, 6518–6524 (2011).