Breaking C-H Bonds to Make Methanol from Methane
Scientific Achievement
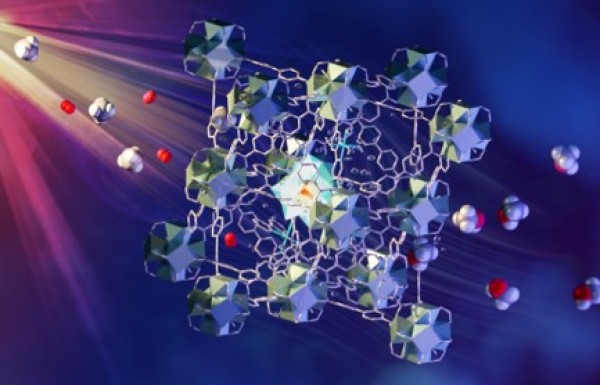
It is shown that strong interactions between methane molecules and mono-iron-hydroxyl sites in a metal-organic framework (MOF) weaken C-H bonds, enabling light-driven methane to methanol conversion.
Significance and Impact
The direct photoconversion of methane to methanol, a liquid fuel and carbon feedstock, can provide an economical method of reducing greenhouse gas emission from methane.
Research Details
- Inelastic neutron scattering (INS) characterized MOFs with and without adsorbed methane.
- Complementary measurements include x-ray diffraction, NMR, infrared and x-ray absorption spectroscopies and electron paramagnetic resonance.
- INS coupled with density functional theory calculations enabled direct observation of methane binding and determined the structure of the bound functional group.
“Direct Photo-oxidation of Methane to Methanol over a Mono-iron Hydroxyl Site,”
Bing An, Zhe Li, Zi Wang, Xiangdi Zeng, Xue Han, Yongqiang Cheng, Alena M. Sheveleva, Zhongyue Zhang, Floriana Tuna, Eric J. L. McInnes,, Mark. D. Frogley, Anibal J. Ramirez-Cuesta, Louise Natrajan, Cheng Wang, Wenbin Lin, Sihai Yan and Martin Schröder,
Nature Materials (2022) (accepted).
DOI: https://doi.org/10.1038/s41563-022-01279-1




