Products like cosmetics, adhesives, and paints rely on a common key component: gels. Polymer gels, a gel type with unique properties, have piqued the interest of researchers because of their potential uses in medical applications.
Studies have shown that modifying the structures of polymer gels can significantly affect their properties, but it is unclear why. To learn how and why that happens, a team of researchers from the Massachusetts Institute of Technology (MIT) and the Department of Energy’s (DOE’s) Oak Ridge National Laboratory (ORNL) are using neutron scattering to take a deeper look.
Polymer gels are made up of polymer chains with junctions connecting them. As thermodynamic conditions change, the sizes of the junctions change relative to the chains connecting them. The changes can cause the gels to become stronger and develop better response properties. To investigate these relationships, the researchers are using the EQ-SANS instrument at ORNL’s Spallation Neutron Source—a DOE Office of Science User Facility.
“We’re trying to understand the role of the junction size and how the junction interactions contribute to the mechanical properties of the gel,” said Christopher Lam, a postdoctoral research associate at ORNL.
Polymer gels are useful for drug delivery because their structures adapt to changes in their environment. For example, a polymer gel with temperature response properties could flow easily at room temperature but then stiffen in a warmer environment like the human body. These types of gels can help ensure that when a drug is injected into the body, it stays in the area it is meant to impact.
Similarly, polymer gels with pressure response properties can be designed to flow easily while under low pressure in a syringe but then stiffen as the gel is ejected and pressure increases.
Neutrons are good probes of materials like polymer gels, largely because of their sensitivity to hydrogen and its isotope, deuterium. Using a unique technique known as contrast matching, researchers replaced some of the hydrogen atoms in the gel with deuterium, which allowed specific structural components to be highlighted by the neutrons.
Using the EQ-SANS instrument’s new rheo-SANS environment enabled the researchers to subject the gels to shear stress—which is stress parallel to the cross section of the material, like two plates sliding past each other—and observe the corresponding changes in structure.
By comparing how the structures of gels with large junctions and those with small junctions shear and deform, the researchers can begin to understand how the sizes of gel junctions can impact the gel properties. Using their findings, the researchers can find ways to develop improved polymer gels.
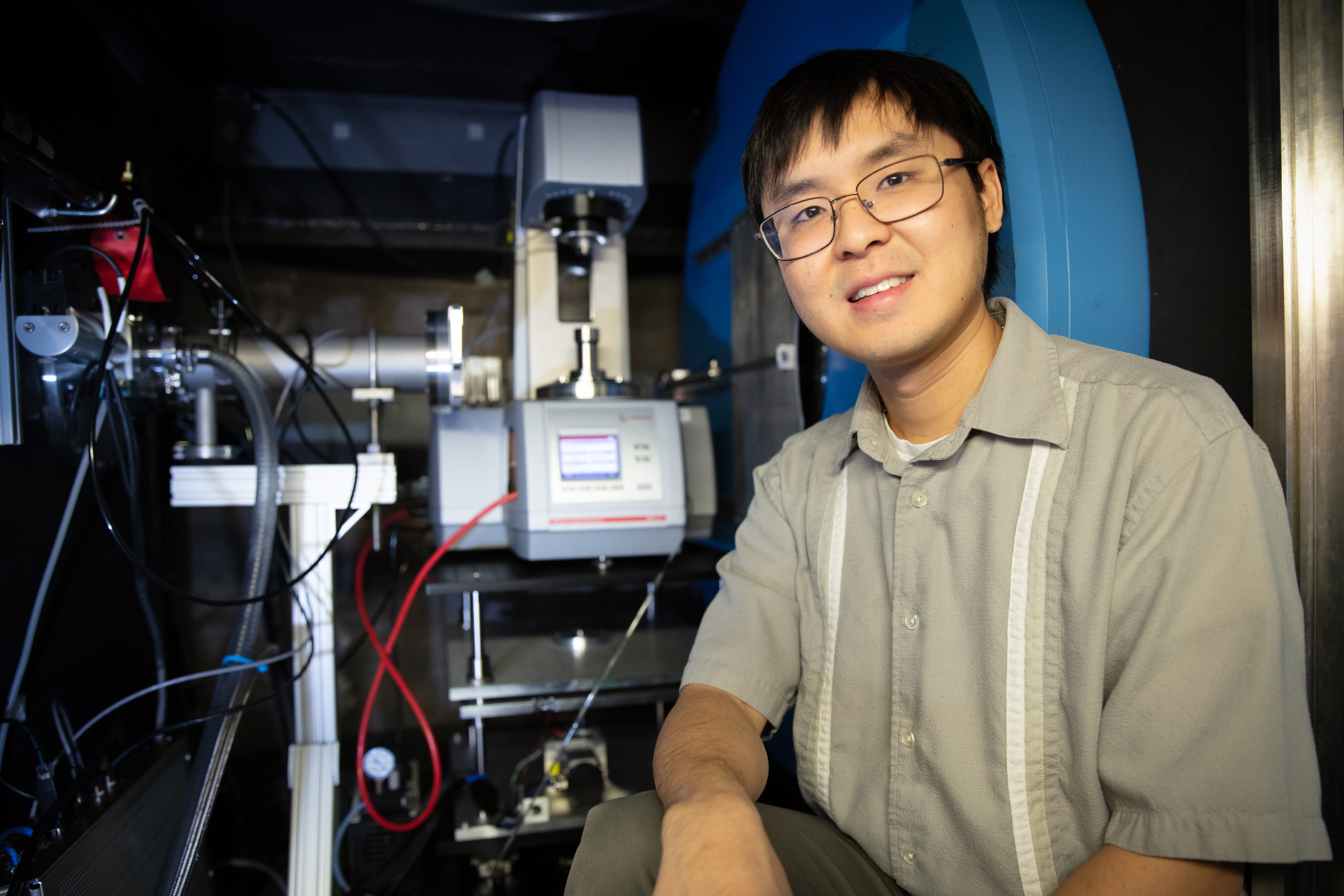
The rheo-SANS environment allowed ORNL’s Christopher Lam to investigate the response properties of
polymer gels at the EQ-SANS beamline at the Spallation Neutron Source. (Credit: ORNL/Genevieve Martin)
“If we have an understanding of the structure of the gels, that basically gives us a better framework,” said Lam. “Then we can say ‘we need this property, this chemical design, and this ratio of components and concentration.’
“I’m always trying to find a balance between how much we do fundamentally and how we can think about applying that to something we’ll use. I’m trying to use this fundamental understanding to really design better biomedical gels.”
Other researchers on this experiment include lead principal investigators Bradley D. Olsen of the Department of Chemical Engineering at MIT, ORNL’s Wei-Ren Chen, and Michelle Calabrese, a postdoctoral researcher at MIT. The research is supported by DOE’s Office of Science.
SNS is a DOE Office of Science User Facility. UT-Battelle LLC manages ORNL for the DOE Office of Science. DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.—by Josh Witt







