Site Specific Deuteration of a Cyclohexene Complex
Scientific Achievement
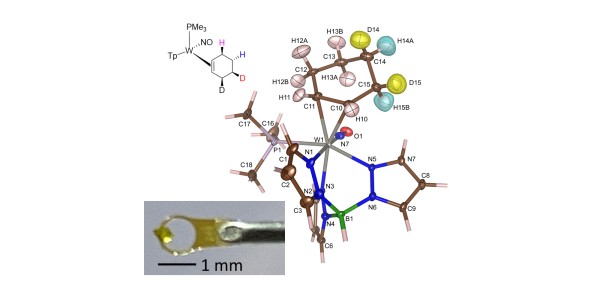
Partially deuterated benzene can be converted to cyclohexene via tungsten binding, with precise control of the position of the deuterium atoms on the cyclohexene ring.
Significance and Impact
The methodology opens new pathways for the preparation of new pharmacologically active compounds where the strategic replacement of hydrogen with deuterium can improve the efficacy and safety of a drug.
Research Details
- The samples were characterized by X-ray diffraction, proton and carbon nuclear magnetic resonance (NMR), high resolution mass spectrometry, and molecular rotational resonant spectroscopy.
- Identity of stereoisotopomers was inferred by proton NMR.
- Neutron diffraction on a small (0.092 mg) single crystal validated the stereochemical assignments of deuterium atom positions for one of the d2 isotopologues.
“Preparation of Cyclohexene Isotopologues and Stereoisotopomers from Benzene”
Jacob A. Smith, Katy B. Wilson, Reilly E. Sonstrom, Patrick J. Kelleher, Kevin D. Welch, Emmit K. Pert, Karl S. Westendorff, Diane A. Dickie, Xiaoping Wang, Brooks H. Pate, and W. Dean Harman
Nature, 581, 288–293 (2020)
DOI: https://doi.org/10.1038/s41586-020-2268-y



