Breaking H-H Bonds for Biomass Conversion
Scientific Achievement
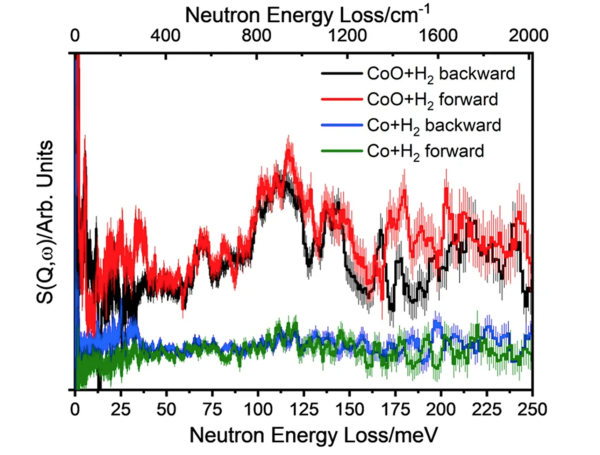
The catalyst Co@ CoO (i.e. Co-core with CoO surface shell) reacts with H2 gas forming Co-H and Co-O-H species, providing direct evidence of heterolytic dissociation of H2.
Significance and Impact
The precious-metal-free Co@ CoO catalyst developed in this work shows excellent activity for hydrodeoxygenation of lignin compounds, a key reaction for biofuel production.
Research Details
- Inelastic neutron scattering (INS) characterized various catalysts before and after reactions with H2 gas at a moderate temperature of 130 °C.
- Samples were also characterized by electron microscopy and X-Ray photoelectron spectroscopy.
- The active site is found to be the CoO shell, not the Co core.
- Density Functional Theory (DFT) calculations supported the conclusions.
“A unique Co@ CoO catalyst for hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to 2,5-dimethylfuran”
Shuang Xiang, Lin Dong, Zhi-Qiang Wang, Xue Han, Luke L. Daemen, Jiong Li, Yongqiang Cheng, Yong Guo, Xiaohui Liu, Yongfeng Hu, Anibal J. Ramirez-Cuesta, Sihai Yang, Xue-Qing Gong & Yanqin Wang
Nature Communications 13, 3657 (2022). DOI: https://doi.org/10.1038/s41467-022-31362-9





