Surface Hydrogen Dominates Ammonia Synthesis Catalyzed by Electride
Scientific Achievement
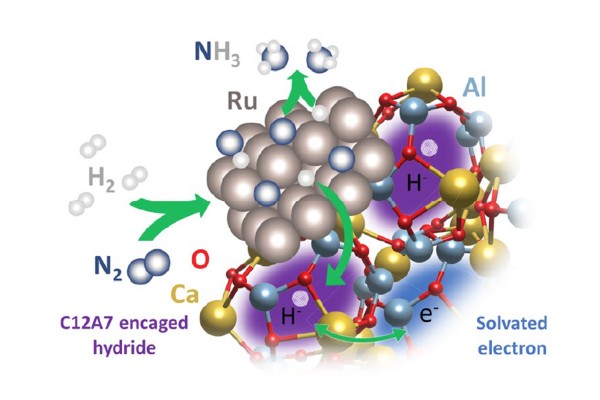
It is shown that the reactive species involved in ammonia synthesis over Ru/C12A7 electride catalysts is surface adsorbed hydrogen, not encaged hydrogen.
Significance and Impact
The results provide a better understanding of the mechanism of ammonia synthesis and N2 activation using electride catalysts, potentially leading to more efficient catalysis of many difficult hydrogenation reactions.
Research Details
- Ru particles supported on a calcium aluminum oxide (C12A7) electride activate N2 to combine with H producing NH3.
- In situ neutron diffraction and spectroscopy combined with density functional theory calculations show the presence of stable encaged hydrides; they do not actively participate in the reaction.
- In contrast to the currently accepted view of the importance of encaged hydrogen, this shows that surface H plays a major role.
- Steady state isotopic transient kinetic analysis (SSITKA) was used to further characterize the reaction mechanism.
“Nature of Reactive Hydrogen for Ammonia Synthesis over a Ru/C12A7 Electride Catalyst”
James Kammert, Jisue Moon, Yongqiang Cheng, Luke Daemen, Stephan Irle, Victor Fung, Jue Liu, Katharine Page, Xiaohan Ma, Vincent Phaneuf, Jianhua Tong, Anibal Ramirez-Cuesta, Zili Wu,
Journal of the American Chemical Society, 142, 7655 (2020). DOI: https://pubs.acs.org/doi/10.1021/jacs.0c02345







