Low-Barrier Hydrogen Bonds Improve Enzyme Interaction
January 17, 2020
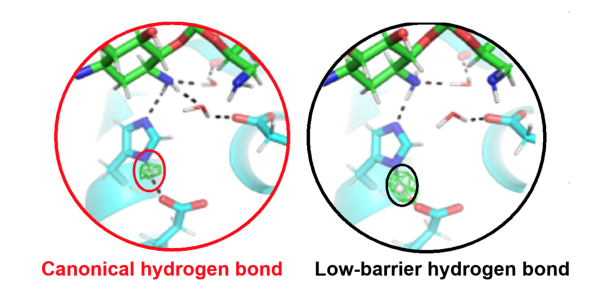
Active site of an antibiotic inactivating enzyme determined with neutron crystallography. In the catalytic triad, the position of the hydrogen atom (circled) controls enzyme specificity and activity. When a low-barrier hydrogen bond is present (right),
Scientific Achievement
The work revealed how a low-barrier hydrogen bond (LBHB) enhances enzyme catalysis 30-fold more than when a canonical hydrogen bond is present.
Significance and Impact
This is the first report of an LBHB and a canonical hydrogen bond being observed in the same active site and a direct demonstration of the catalytic enhancement proposed for LBHBs 30 years ago.
Research Details
- Neutron diffraction was used to differentiate structural details of five antibiotics invisible to X-ray diffraction.
- A single hydrogen atom involved in the catalytic enhancement was directly observed with neutron diffraction.
"Low-Barrier and Canonical Hydrogen Bonds Modulate Activity and Specificity of a Catalytic Triad,"
P. Kumar, P.K. Agarwal, M.B. Waddell, T. Mittag, E.H. Serpersu, M.J. Cuneo,
Angewandte Chemie International Edition, 131 (2019), DOI: https://doi.org/10.1002/anie.201908535



