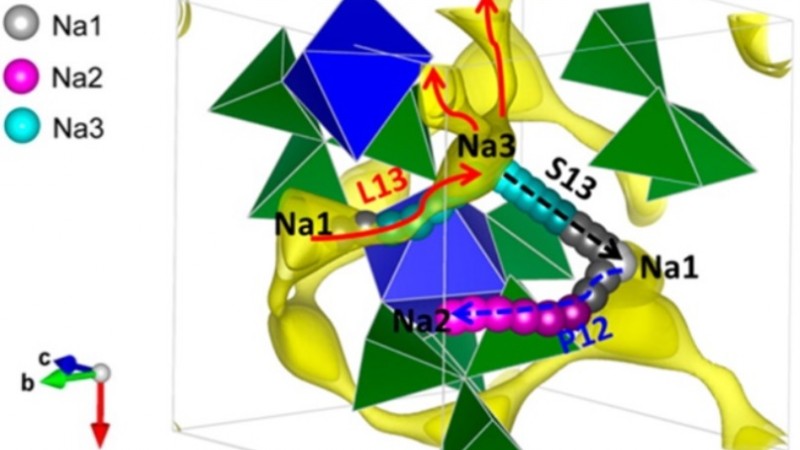
A comprehensive study of Na3TiP3O9N as a cathode material for sodium-ion batteries shows Na+ diffusion pathway to be 3-dimensional and isotropic with very good three-dimensional Na-ion mobility at room temperature in this compound. This Na-ion mobility allows Na3TiP3O9N to function as a cathode in rechargeable Na-ion batteries. The volume change during cycling is exceptionally small (< 1%), offering advantages in designing batteries with long lifetimes. Furthermore, the isotropic three-dimensional diffusion pathway of ions in this framework is ideal for solid state electrolytes, which can enable higher energy densities when incorporated into batteries.
Neutron diffraction studies at NOMAD and POWGEN and X-ray Powder Diffraction were used to study the structures of pristine and de-intercalated materials. Bond valence and DFT calculations were used to determine the Na-ion mobility and diffusion pathway through the lattice. Research supported as part of the Northeastern Center for Chemical Energy Storage (NECCES), an Energy Frontier Research Center funded by the Office of Basic Energy Sciences, US Department of Energy, including matching support from NYSTAR-NYSDED.
Work performed at the Oak Ridge National Laboratory Spallation Neutron Source’s NOMAD and POWGEN instruments was supported by the Scientific User Facilities Division, BES, DOE.
J. Liu, D. Chang, P. Whitfield, Y. Janssen, X. Yu, Y. Zhou, J. Bai, J. Ko, K.-W. Nam, L. Wu, Y. Zhu, M. Feygenson, G. G. Amatucci, A. Van der Ven, X.-Q. Yang, and P. G. Khalifah. "Ionic conduction in cubic Na3TiP3O9N, a secondary Na-ion battery cathode with extremely low volume change." Chemistry of Materials 26 (2014): 3295–3305. http://dx.doi.org/10.1021/cm5011218