Quantum Crystallography Deciphers Mechanistic Pathways to Efficient CO₂ Capture
Scientific Achievement
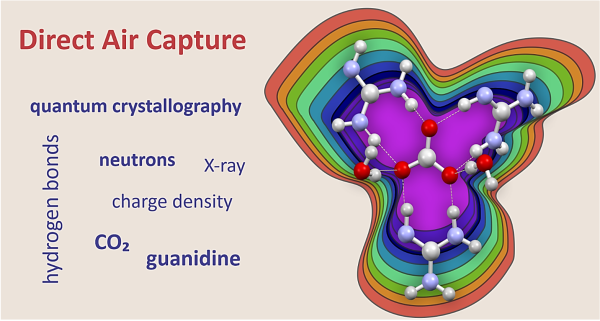
Established a direct experimental framework for linking electron density distributions to intermolecular interactions in a guanidinium carbonate Direct Air Capture material, enabling insight into proton dynamics and interaction polarization.
Significance and Impact
Work reveals how hydrogen-bond polarization and charge separation stabilize CO2-containing crystal phases, providing a rational design strategy for optimizing sorbents with improved CO2 capture efficiency and reduced energy demands.
Research Details
- Quantum crystallography integrating x-ray and neutron diffraction
- Analysis of two crystalline direct air capture phases (P1, P3)
- Identification of polarization-assisted hydrogen bonds (PAHBs)
- Greater binding strength and lattice stability observed in P3
“Intermolecular Interactions in Direct Air Capture Materials: Insights from Charge Density Analysis,” Journal of the American Chemical Society (2025)
DOI: https://doi.org/10.1021/jacs.5c01946





