Summarization of Strong Hydrogen Bond Under High Pressure in Bihydroxide-Ion-Containing NaCu2(SO4)2·H3O2
Scientific Achievement
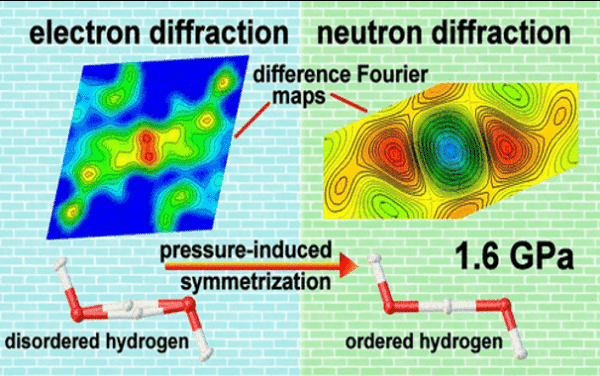
A second-order phase transition was found in bihydroxide anions of [NaCu2(SO4)2·H3O2] that hydrogen bond (HB) symmetrized at the lowest pressure reported so far among inorganic compounds
Significance and Impact
This research offers important insights to understanding the process of water transport to the deeper part of Earth’s mantle and superconductivity in hydrogenous superconductor systems.
Research Details
- Electron and x-ray diffraction with diamond anvil cell and neutron diffraction data with clamp cell solved the atomic displacements.
- Neutron scattering indicates the HBs at disordered H sites become symmetrized at 1.6 GPa, verifying the result from electron diffraction experiments and XRD data analysis with an aspherical atom model.
“Symmetrization of Strong Hydrogen Bond under High Pressure in Bihydroxide-Ion-Containing NaCu2(SO4)2·H3O2 Revealed by Experimental Charge Density, Single-Crystal Electron Diffraction, and Neutron Diffraction Studies, , J. Am. Chem. Soc., 147, 30, 26830–26843, (2025).
https://pubs.acs.org/doi/10.1021/jacs.5c08310



