A team led by the U.S. Department of Energy's Oak Ridge National Laboratory has unlocked the enzymatic synthesis process of rare sugars, which are useful in developing drugs with low side effects using a process more friendly to the environment.
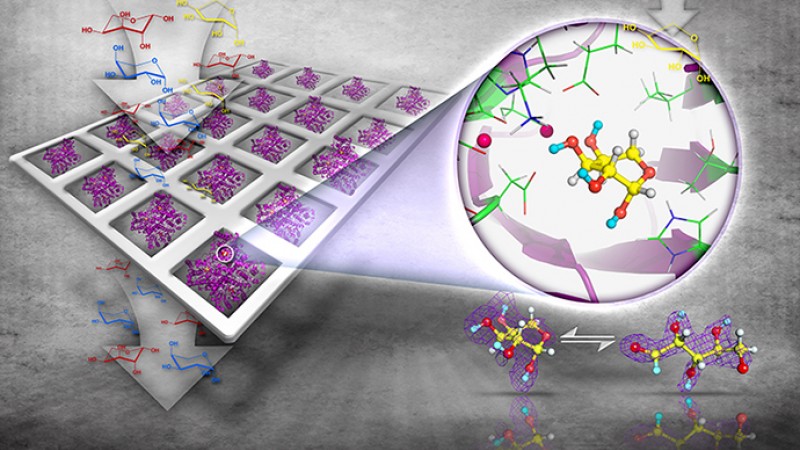
In a paper published in Structure, the research team reported the pioneering use of neutron and X-ray crystallography and high performance computing to study how the enzyme D-xylose isomerase, or XI, can cause a biochemical reaction in natural sugar to produce rare sugars. Unlike drugs made from natural sugar compounds, drugs made from rare sugars do not interfere with cellular processes. As a result, rare sugars have important commercial and biomedical applications as precursors for the synthesis of different antiviral and anti-cancer drugs with fewer side effects.
“The goal of this study is to dramatically improve the performance of enzymes that can be used by the pharmaceutical industry to synthesize drug precursors,” said ORNL’s Andrey Kovalevsky, the lead author of the study. “We’re trying to find a new way to do enzyme design – neutron studies combined with high performance computing could be an elegant means to do that.”
Enzymes speed up reactions in organisms, ultimately making life itself possible, and are increasingly used by industry to synthesize value-added compounds. Biotechnological syntheses are “greener” than other techniques that use heavy metal chemical catalysts and large amounts of organic solvents. However, many natural enzymes are not very well suited for industrial processes. XI, for example, is used effectively for the production of high-fructose corn syrup from starch in the food industry, but its applications in the pharmaceutical industry are limited by its performance. Researchers in the pharmaceutical industry want to engineer mutations in enzymes to improve reactions. But first, they have to understand how the enzymes work.
“We had no idea how the enzyme, D-xylose isomerase, binds its non-physiological substrate – natural sugar L-arabinose,” said Kovalevsky. “You have to know how an enzyme binds its substrate to engineer mutations to improve binding and reaction.”
Using X-ray and neutron crystallography combined with theoretical calculations, the team figured out how the enzyme isomerizes L-arabinose into the rare sugar L-ribulose and then epimerizes the latter into another rare sugar L-ribose. Importantly, L-ribose is the enantiomer, a mirror image, of the ubiquitous D-ribose that is a building block of DNA and RNA.
“We found, completely unexpectedly, that the enzyme binds the substrate L-arabinose –an abundant natural sugar found in plants– in a very high energy geometry in the active site, which explained the xylose isomerase’s poor efficiency with the substrate and provided us with clues on how we can re-engineer it to improve its activity,” said Kovalevsky.
Combining crystallographic observations and computation, the team saw the XI enzyme isomerize the sugar L-arabinose when bound to the active site. Isomerization is the process in which the sugar changes its configuration through a chemical reaction. An enzyme’s active site is the binding place where catalysis is performed on substrates or where inhibitors dock to hinder catalysis. Binding a substrate in a high energy geometry means the efficiency of catalysis would be low, something researchers would like to improve, explained Kovalevsky.
This is the first time researchers have looked at enzymatic synthesis by combining neutrons, X-rays and high performance computing.
“Neutron crystallography gives the location of hydrogen atoms, which is important in enzyme reactions where there’s a lot of shuffling of hydrogen around,” said Kovalevsky. “X-rays can’t see those reactions. But once you have the neutron structures and know the hydrogen positions, then your calculations and theoretical models are much more correct.”
In the past, researchers had to infer the hydrogen atom location from chemical knowledge, which, as experience shows, may be wrong. Now, neutrons show the exact location of the hydrogen atoms so they do not have to guess.
Calculations can be misleading if hydrogens are placed incorrectly, leading in many cases to the wrong inference from calculations about how enzymes function. Combining neutrons, calculations and simulations gives a more thorough view of the enzymes’ mechanisms and a complete look at how enzymes work.
Kovalevsky said future simulations will explore the possibility of tailoring the XI active site to bind lower-energy conformations of L-arabinose to improve catalytic activity.
This research was partially funded through a National Institutes of Health – National Institute of General Medical Sciences consortium between ORNL and DOE’s Lawrence Berkeley National Laboratory (LBNL). The work was conducted in part at the Los Alamos Neutron Science Center, a National Nuclear Security Administration User Facility at DOE’s Los Alamos National Laboratory, and at the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility at LBNL.
UT-Battelle manages ORNL for the Department of Energy’s Office of Science. DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of the time. For more information, please visit science.energy.gov.