Confined Interlayer Water Explains Fast Proton Intercalation
Scientific Achievement
Confined interlayer water in tungsten oxide hydrates (WO3·nH2O) stabilizes the layered structure, which explains the fast electrochemical proton intercalation found in these materials.
Significance and Impact
The work shows that the introduction of confined fluids into redox-active layered materials provides a new strategy for energy storage with both high power and high energy density.
Research Details
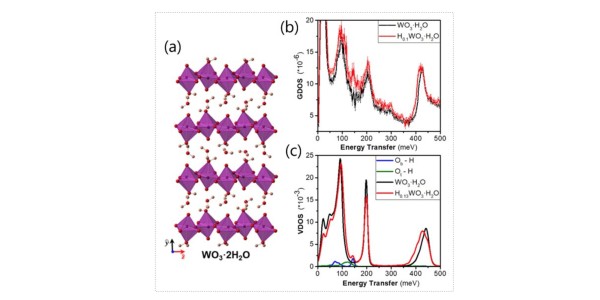
- Quasielastic neutron scattering showed the confined nature of water in WO3·nH2O.
- Inelastic neutron scattering (INS) and density functional theory (DFT) calculations showed that protons occupy bridging oxygen sites.
- X-ray diffraction measurements characterized the operando electrochemically induced structural transformation
“Confined Interlayer Water Promotes Structural Stability for High-Rate Electrochemical Proton Intercalation in Tungsten Oxide Hydrates,”
James B. Mitchell, Natalie R. Geise, Alisa R. Paterson, Naresh C. Osti, Yangyunli Sun, Simon Fleischmann, Rui Zhang, Louis A. Madsen, Michael F. Toney, De-en Jiang, Alexander I. Kolesnikov, Eugene Mamontov, and Veronica Augustyn,
ACS Energy Letters, 4, 2805 (2019). DOI: doi.org/10.1021/acsenergylett.9b02040





