Conformation of Human ISG15 Protein in Complex with SARS-CoV-2 Papain-like Protease
October 11, 2021
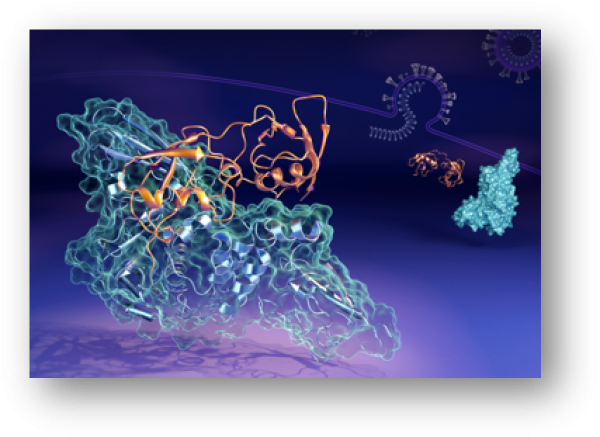
ORNL researchers found the papain-like protease (in orange) can bind to the human interferon-stimulated gene 15 protein (in blue) in multiple ways and shapes.
Scientific Achievement
Investigation of the interaction of papain-like protease (PLpro) with human interferon-stimulated gene 15 (hISG15) showed that the C-terminal domain of hISG15 binds tightly to PLpro, whereas the N-terminal domain adopts different conformational states in solution.
Significance and Impact
The Plpro:hISG15 complex plays a significant role in the interaction of the COVID-19 virus with human antiviral immunity, and the knowledge gained here can help guide the development of new therapeutics targeting this complex.
Research Details
- Small-angle neutron and X-ray scattering (SAX), and computational modelling were used to determine the structure of PLpro:hISG15 complex in solution.
- Small-angle neutron scattering (SANS) using contrast matching resolved the conformation of the ISG15 in the complex.
“Conformational dynamics in the interaction of SARS-CoV-2 papain-like protease with human interferon-stimulated gene 15 protein,”
Wellington Leite, Kevin Weiss, Gwyndalyn Phillips, Qiu Zhang, Shuo Qian, Susan Tsutakawa, Leighton Coates, and Hugh O’Neill
Journal of Physical Chemistry Letters 12, 5608 (2021).
DOI: 10.1021/acs.jpclett.1c00831








