While all viruses have some means of combating the body’s immune system, scientists have been studying how the SARS-CoV-2 coronavirus—the cause of the COVID-19 global pandemic—can evade the immune system in humans.
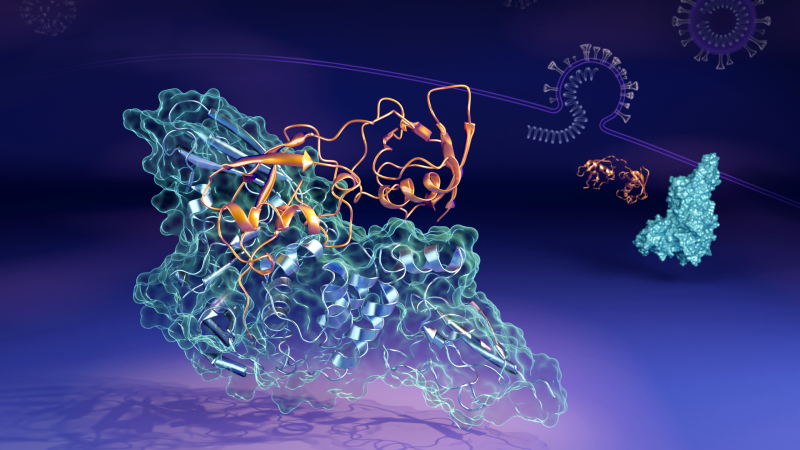
Now scientists working at the US Department of Energy’s (DOE’s) Oak Ridge National Laboratory (ORNL) have revealed the molecular details of how a key protein (the papain-like protease, or “PLpro”) from the virus links up to form a paired-structure, or “complex,” with a human protein named interferon-stimulated gene 15 (ISG15). PLpro strips ISG15 from other human cellular proteins to aid SARS-CoV-2 in evading the immune response. Understanding how the two proteins interact could aid in developing therapeutic drug treatments that prevent its formation and allow a person’s immune system to better fight the invading virus.
The research results, titled “Conformational Dynamics in the Interaction of SARS-CoV-2 Papain Like Protease with Human Interferon-Stimulated Gene 15 Protein,” were published in the Journal of Physical Chemistry Letters.
“In human cells that the virus has infected, the PLpro from the SARS-CoV-2 virus tends to seek out and bind with the ISG15 protein, a key component of the cells’ immune response,” said Hugh O’Neill, leader of ORNL’s Bio-Facilities group and director of the lab’s Center for Structural Molecular Biology. “When PLpro binds to ISG15, it causes the ISG15 to change shape. The key finding is that the ISG15 can assume multiple shapes when it binds to PLpro.”
Using small-angle neutron scattering (SANS) at ORNL’s High Flux Isotope Reactor (HFIR), the researchers were able to study the changes in the complex while they occurred.
“We enhanced the contrast between the PLpro and ISG15 by preparing PLpro in which many of the hydrogen atoms were replaced with deuterium atoms,” said Kevin Weiss, an expert in bio-deuteration. “Neutrons interact differently with deuterium atoms, so this helped us better differentiate between the two proteins.
“We used neutrons to analyze the complex in solution, which better simulates the actual physiological environment of the human body,” said Leighton Coates, instrument systems science and technology manager for ORNL’s Second Target Station. “This allowed us to study the changing shapes of the complex, which other techniques could not have picked up.”
“The information we obtained from our experiments increases our knowledge of how the virus works and will enable us to build more accurate computer models for other scientists to use,” said Wellington Leite, lead author and ORNL postdoctoral researcher. “Researchers will be able to use the model to quickly search for sites on the ISG15 where the PLpro attaches and then try to block those sites.”
Susan Tsutakawa, a biochemist staff scientist at Lawrence Berkeley National Laboratory (Berkeley Lab), obtained small-angle x-ray scattering (SAXS) data on the PLpro-ISG15 complex at the Berkeley Lab’s Advanced Light Source Synchrotron. “In the SAXS studies, we could separate out different complexes in the sample by coupling SAXS with size exclusion chromatography and at the same time, get higher resolution data of the complex’s overall configuration, to complement the SANS studies that revealed the conformations of individual components in the complex,” said Tsutakawa.
The team plans to conduct additional experiments on this type of biological complex to examine how small molecules can block the binding of PLpro to ISG15.
This research was supported by the DOE National Virtual Biotechnology Laboratory, a consortium of DOE national laboratories with funding provided by the Coronavirus CARES Act. Additional support was provided by the DOE Office of Science, the Molecular Biophysics and Integrated Bioimaging Division at Berkeley Lab, and the Center for Structural Molecular Biology at ORNL.
HFIR is a DOE Office of Science user facility. UT-Battelle LLC manages ORNL for the DOE Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit www.energy.gov/science.—by Paul Boisvert